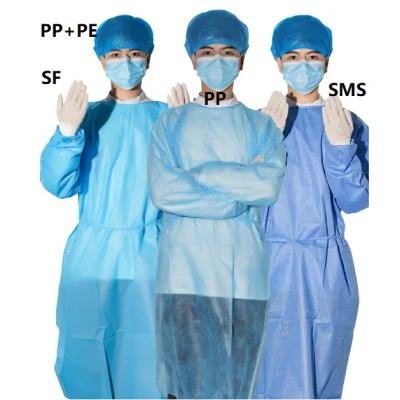
CE Medical Disposable Non-Sterile Reinforced Surgical Gowns Polypropylene Isolation Gown
Basic Info
| Model NO. | BF-SG |
| Materials | PP+PE |
| Color | Blue, White, Red, Yellow etc |
| Transport Package | 100PCS/Carton |
| Specification | all sizes |
| Trademark | BINFEI |
| Origin | China |
| HS Code | 6210103090 |
| Production Capacity | 250000PCS/Day |
Product Description
- Varying levels of protection.
- Ultra-sonically sealed, thermal-bonded or glued sleeve design.
- Freedom of movement with our customer RaglanPlus sleeve design.
- Low-linting, breathable material construction.
- Custom, secure-fit with convenient hook and loop neck closures.
- Preferred knit cuff design.
- Meet the Standard for Flammability of Clothing Textiles CPSC 16 CFR Part 1610.
- Limited to Sterile Items only at this time.

Disposable Surgical Gowns
Disposable surgical gowns are clothes worn by surgeons and other medical staff during operations in the operating room. In addition, a disposable sterile gown protects the patient and healthcare workers from infection during the operation. Moreover, the disposable surgical gowns comply with international surgical gown standards such as EN13795 and AAMI PB70.
As a professional surgical gown manufacturer, we insist on selecting suitable materials, adopting advanced production techniques, and strict quality control systems to provide your medical staff with various types of sterile gowns online. In addition, we have set up production plants and workshops according to GMP requirements, and the purification and asepsis have reached 100000 or 300000 levels of the dust-free environment.
| material | nonwoven fabric&PP+PE&PP&SMS |
| wrist style | elastic wrist, thumb loop |
| Neck style | neck tie, over head style |
| color | blue&white etc. |
| size | 120-140cm etc. |
| inner pack | 100pcs/carton |
| OEM | available |
| certificate | ISO13485&CE |
| Advantage | Cheap/waterproof |

- SMS reinforced gown, the chest and sleeve area are reinforced with an additional layer of SMS material
- Meet ASTM F2407 standard
- Sterile style: Sterilization followed by ISO 11135-1: 2007 standard
- Raglan sleeves, hook and loop neck closures, and tie waist closures
- AAMI Level 3 at the reinforced area
- At the non-reinforced area
- Material: 100 % SMS
- Fabric weight: 45gsm
- Color: Blue
- Size: M, L, XL, XXL
- Packing: 25pcs/ carton
- Knitted cuff type
- PP+PE
- Meet ASTM F2407 standard
- Sterile style: Sterilization followed by ISO 13485 standard
- Raglan sleeves, hook and loop neck closures, and tie waist closures
- AAMI Level 3 at the reinforced area
- At the non-reinforced area
- Fabric weight: 45gsm
- Color: Blue
- Size: M, L, XL, XXL
- Packing: 100pcs/ carton
- Knitted cuff type
- PP gown
- Lightweight, fluid-repelling polypropylene material provides basic protection for the myriad of challenges faced by staff.
- Generously sized for full coverage and flexibility.
- Available with classic neck / waist ties and elastic wrists or over-the-head style neck with thumb loop wrists.
Detailed Photos
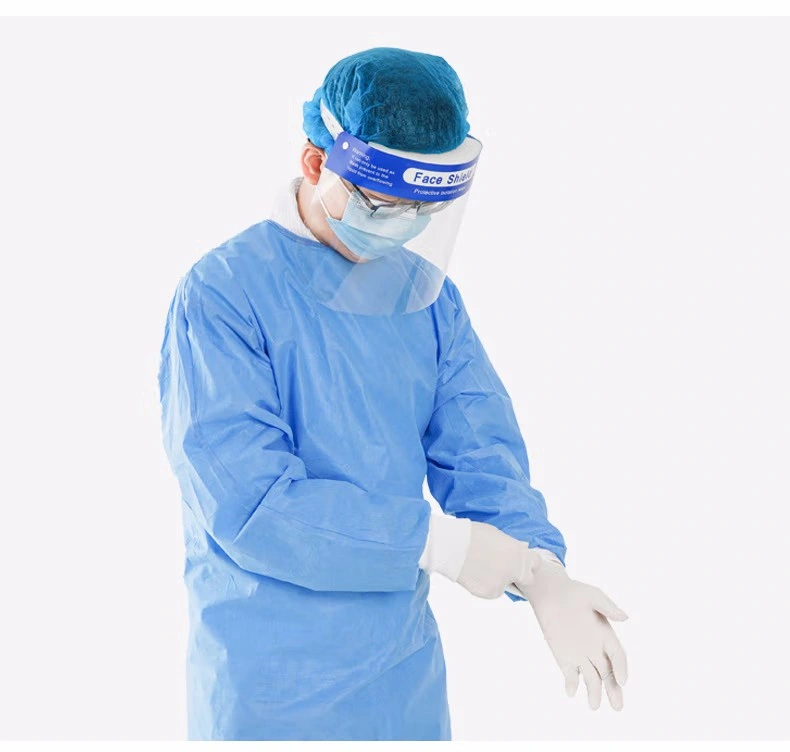


Regards to Surgical Gown
According to FDA, A surgical gown is regulated by the FDA as a Class II medical device that requires a 510(k) premarket notification. A surgical gown is a personal protective garment intended to be worn by health care personnel during surgical procedures to protect both the patient and health care personnel from the transfer of microorganisms, body fluids, and particulate matter.
Because of the controlled nature of surgical procedures, critical zones of protection have been described by national standards. As referenced in Figure 1: the critical zones include the front of the body from the top of shoulders to knees and the arms from the wrist cuff to above the elbow. Surgical gowns can be used for any risk level (Levels 1-4). All surgical gowns must be labeled as surgical gowns.
Source FDA
Regards to Surgical Isolation Gown
Surgical isolation gowns are used when there is a medium to high risk of contamination and a need for larger critical zones than traditional surgical gowns. Surgical isolation gowns are regulated by the FDA as a Class II medical device that requires a 510(k) premarket notification.
All areas of the surgical isolation gown except bindings, cuffs, and hems are considered critical zones of protection and must meet the highest liquid barrier protection level for which the gown is rated. All seams must have the same liquid barrier protection as the rest of the gown. Additionally, the fabric of the surgical isolation gown should cover as much of the body as is appropriate for the intended use.
Source FDA
CertificationsCompany ProfileWE HAVE OWNED OURSELVES FACTORY IN 2015, THERE ARE 100 SQUARE METERS IN HEFEI OFFICE AND 100000 SQUARE METERS IN SHAN DONG FACTORYIS ONE OF THE MOST PROFESSIONAL DISPOSABLE DENTAL PRODUCTS' SUPPLIER IN CHINA.For now, we can supply :sterilization pouches & rolls, dental bibs, saliva ejectors, mixing tip, face masks, surgical gowns, face shield, dental kit, cheek tray, plishing brush, micro brush applicator, barrier film, alcohol pad swab, oral foam swab, mob clip cap, surgical blade,Irrigation needle, anaesthesia
needle, Ortho prosthesis box, cotton roll, air water tip, dental Dappen cup, Endo measure block, mixing tip, Prophylaxis Brushes, cheek retractors, mouth gags open my mouth, Impression tray, Dental Wedge, Mixing spatula, Dental kits(more in one), Medical face shield, Goggles, spectacle face visor, all kind of shoe covers and gowns, Plastic Tray, alginate, Endo Ring Foam Refill .
Our AdvantagesLooking forward to cooperating with you in the future ......






